The first companion diagnostic tests were designed for therapeutics used in treating breast cancers resulting from over-expression of the HER2/neu receptor. A monoclonal antibody marketed as Herceptin by Genentech (now Roche) targeted the HER2 receptor. Over-expression of the HER2 protein was determined by examining formalin-fixed, paraffin-embedded (FFPE) breast cancer tissue specimens using immunohistochemistry (IHC) or in situ hybridization (ISH) based companion diagnostic. This was back almost 17 years ago at the turn of the century at the dawn of the human Genome revolution. Today, there are at least 36 FDA cleared companion diagnostics in the market, targeting at least nine different types of tumors. Over 17 years this number is small, however about 50% of these came during the past three years. According to market reports, the companion diagnostics market will grow at a CAGR of 20.1%, making it the hottest molecular diagnostics growth segment. This article will discuss the current state of affairs for companion diagnostics and what the future holds.
In recent years, the the FDA issued “Guidance for Industry: In Vitro Companion Diagnostic Devices,” and released the draft guidance, “Principles for Codevelopment of an In Vitro Companion Diagnostic Device with a Therapeutic Product.” These documents are a resource for drug developers to help decide the need for companion diagnostics earlier in the drug development process and serve as a guidance document for its co-development. In 2017, the FDA has approved four companion diagnostics as of August. These include:
- Abbott’s RealTime IDH2 with Celgene’s Idhifa® for treatment of acute myeloid leukemia (AML) patients with IDH2 mutation
- Illumina’s Praxis Extended Ras Panel with Amgen’s Vectibix® for the treatment of colorectal cancer in patients with 56 specific mutations in RAS genes
- ThermoFisher Scientific’s Oncomine Dx Test with Novartis’ Tafinlar® in combination with Mekinist® for metastatic non-small cell lung cancer (NSCLC) based on BRAF V600E mutation
- Invivoscribe Technologies’ LeukoStrat® CDx FLT3 Mutation Assay with Novartis Midostaurin for AML patients with mutations in FLT3 gene
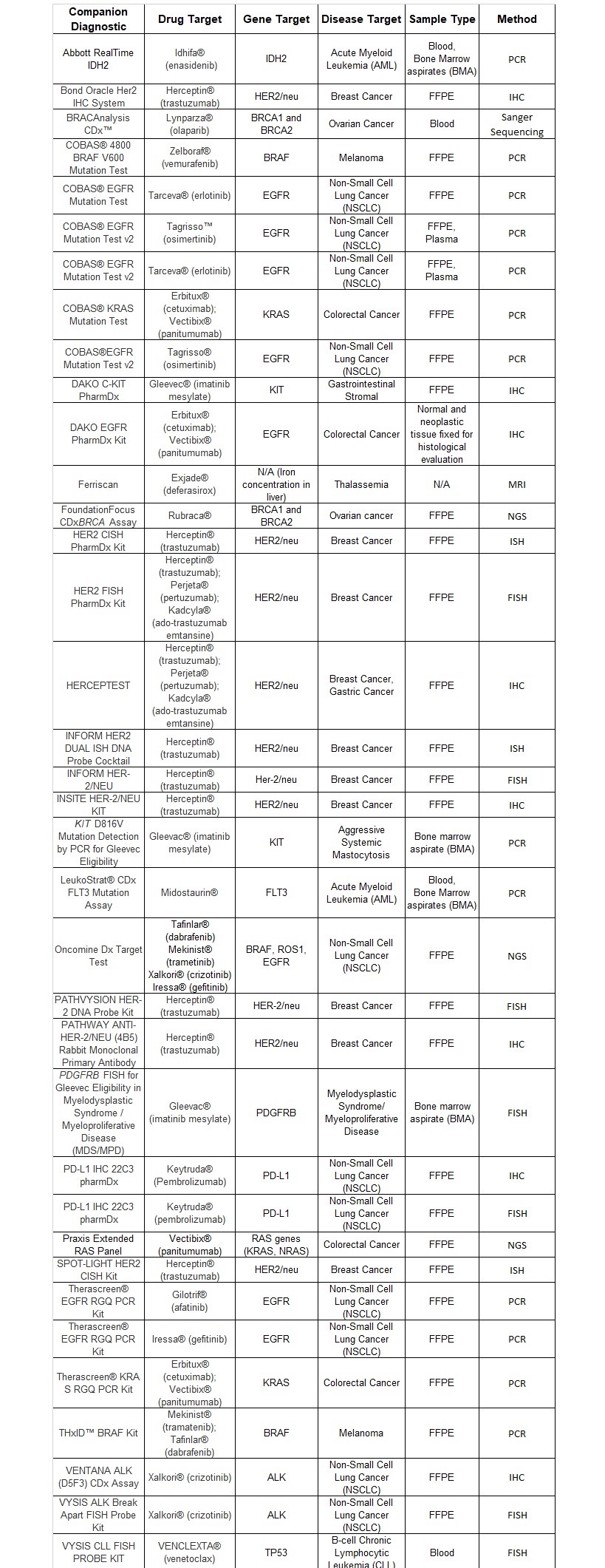
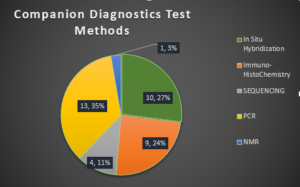
All of these tests employ either PCR or Next Generation Sequencing (NGS) methods. The table at the end of this article has a listing of companion diagnostics and their corresponding therapeutic, disease indication, gene target, sample type, and method of testing.
Majority of these tests require use of FFPE tissue or bone marrow aspirate (BMA), however there are few tests that use blood or plasma as a sample type. While FFPE tissue is challenging to handle, over the last several years technological progress has improved the quality and quantity of nucleic acid extracted from this sample type. The first companion diagnostics employed ISH and IHC; the newer companion diagnostics are using PCR and DNA sequencing based test methods, which wouldn’t have been possible without the human genome revolution. PCR and DNA sequencing methods have improved the sensitivity of newer assays, requiring very little sample for detection, which is often a challenge when working with tissue biopsies.
Companion diagnostics has made an impact on the 4Ps – the Pharma companies, the patients, the payers, and the policy regulators – creating a win-win situation for all. Pharma is now able to selectively identify patients who are most likely to benefit from the clinical trial, improving the chances of drug demonstrating effectiveness and ultimately time to market. Patients are able to identify the right treatment that would work for them, avoiding the emotional pain of going through lengthy treatments that may not work or worse leave them with adverse effects. Payers see improved returns on investment by restricting insurance coverage policy based on results from the companion test, keeping rising health costs in check. Finally, the policy regulators see the potential for minimizing adverse events during clinical trials, fulfilling its mission of keeping the public safe.
The companies that have FDA approved companion diagnostics today are Abbott Molecular, ARUP Laboratories, Biogenex Laboratories, bioMerieux, Agilent, Foundation Medicine, Illumina, InvivoScribe Technologies, Leica Biosystems, ThermoFisher Scientific, Myriad Genetics, Qiagen, Resonance Health Analysis Services, and Roche. One would notice that majority of these companies are not Pharma companies. This may imply that Pharma is not necessarily planning to bring Companion Diagnostics in-house. However, if this market continues to grow at 20% per year and potentially more, this could change and lead to more investment or M&A activity in this sector. Two years ago Roche bought a significant stake in Foundation Medicine, a maker of companion diagnostics targeting tumors. Additionally, today the focus is on bringing effective therapies to market. Just this month alone, Ignyta has announced plans to seek approval for Entrectinib in ROS1-Positive lung cancer and Foundation Medicine is advancing a blood-based version of its mutational burden test as a companion to Tecentriq for treatment of NSCLC.
However, there are many drug targets sitting on the shelf which could benefit from a companion diagnostic approach. The race for rescuing old failed drug candidates using companion diagnostics is just beginning. And the human genome revolution continues to make a positive impact.
Table 1: Listing of Companion Diagnostics:
Additional articles by the author can be found here.