FDA CFR 21 Part 820
Subpart A: General Provisions
Subpart B: Quality System Requirements
Subpart C: Design Controls
Subpart D: Document Controls
Subpart E: Purchasing Controls
Subpart F: Identification and Traceability
Subpart G: Production and Process Controls
Subpart H: Acceptance Activities
Subpart I: Nonconforming Product
Subpart J: Corrective and Preventative Action
Subpart K: Labeling and Packaging Control
Subpart L: Handling, Storage, Distribution, and Installation
Subpart M: Records
Subpart N: Servicing
Subpart O: Statistical Controls
For latest Electronic Code of Federal Regulations
FDA Guide to Inspection of Quality Systems
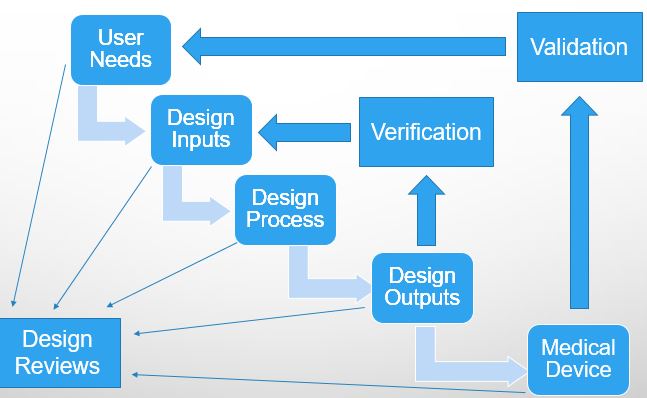
Product Development Road Map
Voice of the Customer
Design History File
⇓
User Needs
Design Inputs
Design Outputs
⇓
Verification of Design Inputs
Validation of User Needs
⇓
Device Master Record
Regulatory Submission/Approval
⇓
Design Transfer
Limited & Full Product Launch